使用者:Schenad/反應-擴散系統

反應-擴散系統(reaction–diffusion system)是一類數學模型的統稱。這類模型和許多物理現象相關:最常見的是一種或多種化學物質的濃度在空間分布上的變化。在這個過程中,局部的化學反應使化學物質相互轉變;擴散作用使物質向四周分散運動。反應-擴散系統類比的是在反應和擴散這兩種不同機制的競爭下,物質在空間分布上的具體行為。
反應-擴散系統不僅被應用到化學研究中,還可以描述沒有化學反應參與的動力學過程,在生物學、地質學、物理學(中子擴散理論)和生態學領域中都有相應的例子。
在數學上,反應-擴散方程式是半線性拋物偏微分方程式,一般形式為:
其中,q(x, t) 表示未知的向量函數,D是品質擴散率的對角矩陣,R 表示所有的局域性反應。
反應-擴散方程式的解行為五花八門,其中包括行波的形成、類波現象,以及自組裝圖案(條紋、六邊形或是更複雜的結構,例如耗散孤子)。
單組分反應-擴散方程式[編輯]
最簡單的反應-擴散方程式是一維的:
若去掉式子中的反應項 ,此方程式化為普通的擴散方程式,即菲克第二定律。若 ,則方程式化為費雪方程式[1],最初用於描述人口的流動[2]。若 ,則變為 Newell–Whitehead-Segel 方程式,用於描述瑞立-貝納德對流[3][4]。更為一般的情況是澤爾多維奇方程式: 且 ,在燃燒理論中出現[5];它的特例 有時也被稱作澤爾多維奇方程式[6]。
演化方程式又可以寫作變碎形式:
於是,藉助以下的泛函,給出了一個單調遞減的自由能(free energy) :
其中的位能 滿足

In systems with more than one stationary homogeneous solution, a typical solution is given by travelling fronts connecting the homogeneous states. These solutions move with constant speed without changing their shape and are of the form u(x, t) = û(ξ) with ξ = x − ct, where c is the speed of the travelling wave. Note that while travelling waves are generically stable structures, all non-monotonous stationary solutions (e.g. localized domains composed of a front-antifront pair) are unstable. For c = 0, there is a simple proof for this statement:[7] if u0(x) is a stationary solution and u = u0(x) + ũ(x, t) is an infinitesimally perturbed solution, linear stability analysis yields the equation
With the ansatz ũ = ψ(x)exp(−λt) we arrive at the eigenvalue problem
of Schrödinger type where negative eigenvalues result in the instability of the solution. Due to translational invariance ψ = ∂x u0(x) is a neutral eigenfunction with the eigenvalue λ = 0, and all other eigenfunctions can be sorted according to an increasing number of knots with the magnitude of the corresponding real eigenvalue increases monotonically with the number of zeros. The eigenfunction ψ = ∂x u0(x) should have at least one zero, and for a non-monotonic stationary solution the corresponding eigenvalue λ = 0 cannot be the lowest one, thereby implying instability.
To determine the velocity c of a moving front, one may go to a moving coordinate system and look at stationary solutions:
This equation has a nice mechanical analogue as the motion of a mass D with position û in the course of the "time" ξ under the force R with the damping coefficient c which allows for a rather illustrative access to the construction of different types of solutions and the determination of c.
When going from one to more space dimensions, a number of statements from one-dimensional systems can still be applied. Planar or curved wave fronts are typical structures, and a new effect arises as the local velocity of a curved front becomes dependent on the local radius of curvature (this can be seen by going to polar coordinates). This phenomenon leads to the so-called curvature-driven instability.[8]
雙組分反應-擴散方程式[編輯]
Two-component systems allow for a much larger range of possible phenomena than their one-component counterparts. An important idea that was first proposed by Alan Turing is that a state that is stable in the local system can become unstable in the presence of diffusion.[9]
A linear stability analysis however shows that when linearizing the general two-component system
of the stationary homogeneous solution will satisfy
Turing's idea can only be realized in four equivalence classes of systems characterized by the signs of the Jacobian R′ of the reaction function. In particular, if a finite wave vector k is supposed to be the most unstable one, the Jacobian must have the signs
This class of systems is named activator-inhibitor system after its first representative: close to the ground state, one component stimulates the production of both components while the other one inhibits their growth. Its most prominent representative is the FitzHugh–Nagumo equation
with f (u) = λu − u3 − κ which describes how an action potential travels through a nerve.[10][11] Here, du, dv, τ, σ and λ are positive constants.
When an activator-inhibitor system undergoes a change of parameters, one may pass from conditions under which a homogeneous ground state is stable to conditions under which it is linearly unstable. The corresponding bifurcation may be either a Hopf bifurcation to a globally oscillating homogeneous state with a dominant wave number k = 0 or a Turing bifurcation to a globally patterned state with a dominant finite wave number. The latter in two spatial dimensions typically leads to stripe or hexagonal patterns.
- Subcritical Turing bifurcation: formation of a hexagonal pattern from noisy initial conditions in the above two-component reaction-diffusion system of Fitzhugh-Nagumo type.
-
Noisy initial conditions at t = 0.
-
State of the system at t = 10.
-
Almost converged state at t = 100.
For the Fitzhugh-Nagumo example, the neutral stability curves marking the boundary of the linearly stable region for the Turing and Hopf bifurcation are given by
If the bifurcation is subcritical, often localized structures (dissipative solitons) can be observed in the hysteretic region where the pattern coexists with the ground state. Other frequently encountered structures comprise pulse trains (also known as periodic travelling waves), spiral waves and target patterns. These three solution types are also generic features of two- (or more-) component reaction-diffusion equations in which the local dynamics have a stable limit cycle[12]
- Other patterns found in the above two-component reaction-diffusion system of Fitzhugh-Nagumo type.
-
Rotating spiral.
-
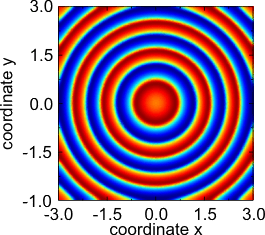
Target pattern.
-
Stationary localized pulse (dissipative soliton).
三組分或多組分反應-擴散方程式[編輯]
For a variety of systems, reaction-diffusion equations with more than two components have been proposed, e.g. as models for the Belousov-Zhabotinsky reaction,[13] for blood clotting[14] or planar gas discharge systems.[15]
It is known that systems with more components allow for a variety of phenomena not possible in systems with one or two components (e.g. stable running pulses in more than one spatial dimension without global feedback),.[16] An introduction and systematic overview of the possible phenomena in dependence on the properties of the underlying system is given in.[17]
實際應用和普遍性[編輯]
In recent times, reaction–diffusion systems have attracted much interest as a prototype model for pattern formation. The above-mentioned patterns (fronts, spirals, targets, hexagons, stripes and dissipative solitons) can be found in various types of reaction-diffusion systems in spite of large discrepancies e.g. in the local reaction terms. It has also been argued that reaction-diffusion processes are an essential basis for processes connected to morphogenesis in biology[18] and may even be related to animal coats and skin pigmentation.[19][20] Other applications of reaction-diffusion equations include ecological invasions,[21] spread of epidemics,[22] tumour growth[23][24][25] and wound healing.[26] Another reason for the interest in reaction-diffusion systems is that although they are nonlinear partial differential equations, there are often possibilities for an analytical treatment.[27][28][29]
實驗驗證[編輯]
迄今為止,對於化學中的反應-擴散系統,控制良好的實驗可由以下三種方式實現:
除了上述的例子之外,像電漿之類的電子輸運系統[35]或是半導體[36]在某種條件之下也可以用反應-擴散系統來描述。
數值處理[編輯]
反應-擴散系統可以用數值分析求解。一些研究論文中可找到不少針對此系統採用的數值處理方法[37]。同時,數值法也被用於相關的複幾何學中[38][39]。
另見[編輯]
反應-擴散方程式的幾個特例[編輯]
參考資料[編輯]
- ^ A. Kolmogorov et al., Moscow Univ. Bull. Math. A 1 (1937): 1
- ^ R. A. Fisher, Ann. Eug. 7 (1937): 355
- ^ A. C. Newell and J. A. Whitehead, J. Fluid Mech. 38 (1969): 279
- ^ L. A. Segel, J. Fluid Mech. 38 (1969): 203
- ^ Y. B. Zeldovich and D. A. Frank-Kamenetsky, Acta Physicochim. 9 (1938): 341
- ^ B. H. Gilding and R. Kersner, Travelling Waves in Nonlinear Diffusion Convection Reaction, Birkhäuser (2004)
- ^ P. C. Fife, Mathematical Aspects of Reacting and Diffusing Systems, Springer (1979)
- ^ A. S. Mikhailov, Foundations of Synergetics I. Distributed Active Systems, Springer (1990)
- ^ A. M. Turing, Phil. Transact. Royal Soc. B 237 (1952): 37
- ^ R. FitzHugh, Biophys. J. 1 (1961): 445
- ^ J. Nagumo et al., Proc. Inst. Radio Engin. Electr. 50 (1962): 2061
- ^ N. Kopell and L.N. Howard, Stud. Appl. Math. 52 (1973): 291
- ^ V. K. Vanag and I. R. Epstein, Phys. Rev. Lett. 92 (2004): 128301
- ^ E. S. Lobanova and F. I. Ataullakhanov, Phys. Rev. Lett. 93 (2004): 098303
- ^ H.-G. Purwins et al. in: Dissipative Solitons, Lectures Notes in Physics, Ed. N. Akhmediev and A. Ankiewicz, Springer (2005)
- ^ C. P. Schenk et al., Phys. Rev. Lett. 78 (1997): 3781
- ^ A. W. Liehr: Dissipative Solitons in Reaction Diffusion Systems. Mechanism, Dynamics, Interaction. Volume 70 of Springer Series in Synergetics, Springer, Berlin Heidelberg 2013, ISBN 978-3-642-31250-2
- ^ L.G. Harrison, Kinetic Theory of Living Pattern, Cambridge University Press (1993)
- ^ H. Meinhardt, Models of Biological Pattern Formation, Academic Press (1982)
- ^ Murray, James D. Mathematical Biology. Springer Science & Business Media. 9 March 2013: 436–450. ISBN 978-3-662-08539-4.
- ^ E.E. Holmes et al, Ecology 75 (1994): 17
- ^ J.D. Murray et al, Proc. R. Soc. Lond. B 229 (1986: 111
- ^ M.A.J. Chaplain J. Bio. Systems 3 (1995): 929
- ^ J.A. Sherratt and M.A. Nowak, Proc. R. Soc. Lond. B 248 (1992): 261
- ^ R.A. Gatenby and E.T. Gawlinski, Cancer Res. 56 (1996): 5745
- ^ J.A. Sherratt and J.D. Murray, Proc. R. Soc. Lond. B 241 (1990): 29
- ^ P. Grindrod, Patterns and Waves: The Theory and Applications of Reaction-Diffusion Equations, Clarendon Press (1991)
- ^ J. Smoller, Shock Waves and Reaction Diffusion Equations, Springer (1994)
- ^ B. S. Kerner and V. V. Osipov, Autosolitons. A New Approach to Problems of Self-Organization and Turbulence, Kluwer Academic Publishers (1994)
- ^ K.-J. Lee et al., Nature 369 (1994): 215
- ^ C. T. Hamik and O. Steinbock, New J. Phys. 5 (2003): 58
- ^ H. H. Rotermund et al., Phys. Rev. Lett. 66 (1991): 3083
- ^ M. D. Graham et al., J. Phys. Chem. 97 (1993): 7564
- ^ A. L. Hodgkin and A. F. Huxley, J. Physiol. 117 (1952): 500
- ^ M. Bode and H.-G. Purwins, Physica D 86 (1995): 53
- ^ E. Schöll, Nonlinear Spatio-Temporal Dynamics and Chaos in Semiconductors, Cambridge University Press (2001)
- ^ S.Tang et al., J.Austral.Math.Soc. Ser.B 35(1993): 223-243
- ^ Isaacson, Samuel A.; Peskin, Charles S. Incorporating Diffusion in Complex Geometries into Stochastic Chemical Kinetics Simulations. SIAM J. Sci. Comput. 2006, 28 (1): 47–74. doi:10.1137/040605060.
- ^ Linker, Patrick. Numerical methods for solving the reactive diffusion equation in complex geometries. The Winnower. 2016.
外部連結[編輯]
- Reaction-Diffusion by the Gray-Scott Model: Pearson's parameterization a visual map of the parameter space of Gray-Scott reaction diffusion.
- A Thesis on reaction-diffusion patterns with an overview of the field
[[Category:数学模型]] [[Category:拋物型偏微分方程]]











![{\displaystyle {\mathfrak {L}}=\int _{-\infty }^{\infty }\left[{\tfrac {D}{2}}\left(\partial _{x}u\right)^{2}-V(u)\right]{\text{d}}x}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c8b1328022ec2404f796e776850c67d1521a16ce)













![{\displaystyle {\begin{aligned}q_{\text{n}}^{H}(k):&{}\quad {\frac {1}{\tau }}+\left(d_{u}^{2}+{\frac {1}{\tau }}d_{v}^{2}\right)k^{2}&=f^{\prime }(u_{h}),\\[6pt]q_{\text{n}}^{T}(k):&{}\quad {\frac {\kappa }{1+d_{v}^{2}k^{2}}}+d_{u}^{2}k^{2}&=f^{\prime }(u_{h}).\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/44e1fc5b936101f66caea7f346e2c29a3b879ef2)